 |
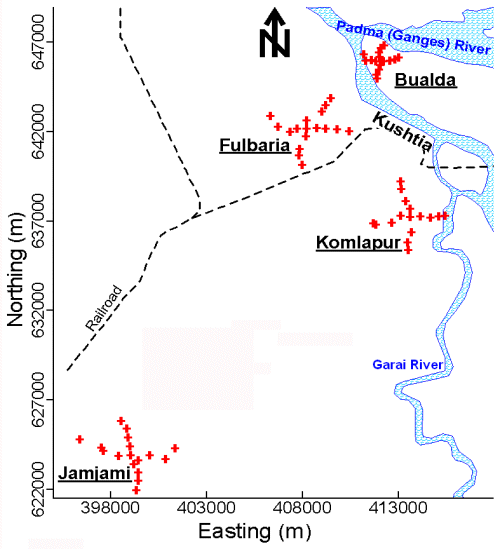
To the extent possible, the sampled tubewells in each neighborhood were distributed at 500-meter intervals along perpendicular axes that radiated in 4 equal lengths from the center (Figure 2). Two samples were collected from the centermost tubewell in each neighborhood. One sample was collected from each of the remaining tubewells. The latitude and longitude of these tubewells were determined using a Garmin Global Positioning System 12 Channel Personal Navigator™. The accuracy of this instrument was approximately 15 meters.
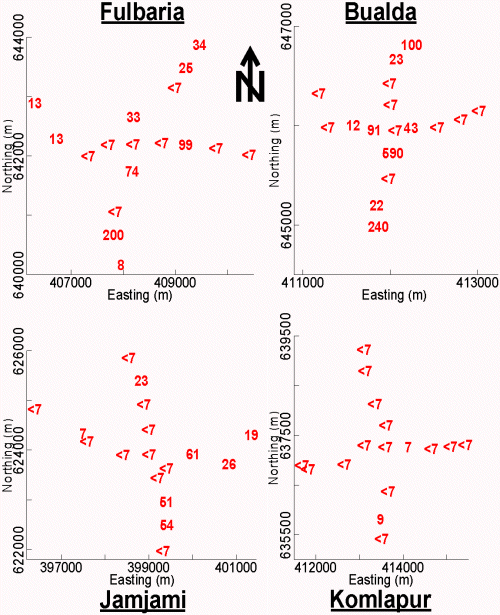 |
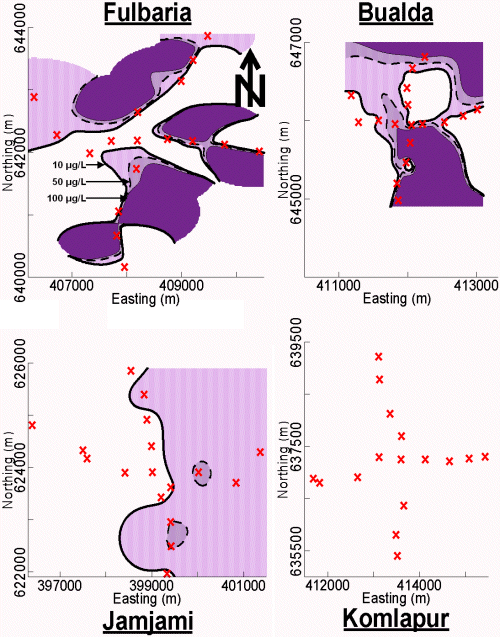
Established collection, preservation, and storage methodologies were used to ensure that each sample was representative of groundwater quality (APHA et al. 1995; Stumm and Morgan 1981). Accordingly, all sampled tubewells were purged by pumping vigorously for 10 minutes (min) immediately before sample collection. All samples were collected directly into polyethylene bottles. These samples were not filtered. Samples were analyzed immediately after collection with pH paper, preserved by acidification to pH < 2 with 5.0 Molar (M) hydrochloric acid (HCl), and stored in ice-packed coolers. The temperature of all stored samples was maintained at 0º to 4º Celsius (C) until immediately before analysis at laboratories in Dubai and Vermont. In Dubai, all of these samples were analyzed for As by both the arsenomolybdate and AgSCSN(CH2CH3)2 methods. The samples were subsequently shipped to Vermont and analyzed for As by GFAAS. Contour maps of the As concentration in tubewell water from these 4 neighborhoods were drawn (Figure 3).
 |
The recovery of a known addition of standard to 8 different samples was used to assess the matrix effects of all 3 methods of determining As. In addition, 3 types of precision were measured for each method from the analysis of 7 different standards (precision of standards), the duplicate analyses of 8 different samples (precision of samples), and the analysis of a known addition of standard to 8 different samples (precision of known additions). The same 8 samples were used to assess the matrix effects and precisions of all 3 methods of determining As (Table 1; APHA et al. 1995).
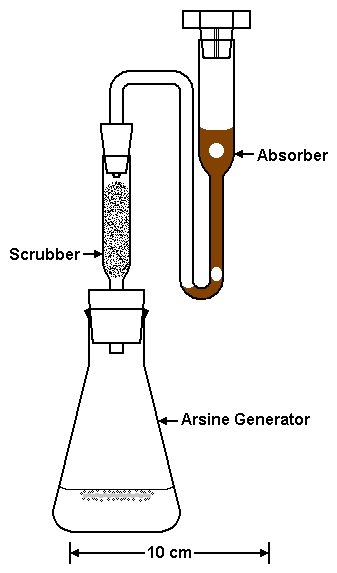 |
Calibrate a test tube to receive 5.00 mL. Pour the liquid from the absorber to this test tube. Use 0.50 mL of distilled water to rinse the residual liquid from the absorber to the test tube. Add 1.00 mL of H2SO4/(NH4)6Mo7O24.4H2O solution to the test tube and mix. Add 0.50 mL of 6.00% (weight/volume) Na2S2O5 solution to the test tube and mix. The Na2S2O5 should change the mixture from deep reddish-brown to faint yellow. The brown color must be eliminated. If necessary, add distilled water to the test tube until its total volume of liquid is 5.00 mL and mix. Add 0.50 mL of 0.20% (weight/volume) SnCl2.2H2O to the test tube, mix, and wait 30 min for the bluish-green arsenomolybdate color to develop. Measure the absorbance at 835 nm.
Confirm that the calibration results obey Beer’s law. That is, test for a higher order polynomial relationship to confirm linearity, and test the null hypothesis that the y-intercept goes through the origin (Neter et al. 1985).
An F-test was used to determine if 2 precisions were equivalent or different. Precision is a standard deviation (Table 1; APHA et al. 1995). Therefore, precision squared is a variance (Snedecor and Cochran 1982). A ratio of these variances is an F-test of the equality of the corresponding precisions (Snedecor and Cochran 1982). Each F-test was evaluated at the 95% confidence level.
Interviewees with As concentrations less than or equal to the 10-µg/L WHO drinking water guideline were informed that their tubewells were safe with respect to this element. In addition, they were informed that their tubewells should be tested for As at least once a year because the concentration of As can change over time (Frisbie et al. 1999; USAID 1997). Finally, they were asked to share their drinking water with those who could not get safe water from their own tubewells.
Interviewees with As concentrations greater than the 10-µg/L WHO drinking water guideline were informed that their tubewells were unsafe with respect to this element. The imminent risk of serious health problems from continuing to drink this water was thoroughly explained. Finally, they were asked to get safe drinking water from their neighbors.